
Play Text-to-Speech:
Cathodic protection (CP) is a corrosion control technique used to protect metal structures from corrosion by converting them into the cathode of an electrochemical cell. CP is commonly applied to steel pipelines, storage tanks, offshore platforms, and other metal infrastructures exposed to corrosive environments such as seawater or soil.
Corrosion occurs because of electrochemical reactions between metal and its environment. In an electrochemical cell, the metal acts as an anode (where oxidation occurs), and it loses electrons, which leads to material deterioration (corrosion). Cathodic protection halts this reaction by ensuring the metal structure becomes a cathode and receives electrons rather than losing them.
This guide will cover the basic theory, Pourbaix diagram, standards, applications, measurement parameters, site inspection readings, and critical factors to ensure effective cathodic protection.
1. Basic Theory of Cathodic Protection
Cathodic protection works by introducing a new anode system that provides electrons to the metal structure, preventing it from corroding. There are two main types of cathodic protection systems:
- Galvanic (Sacrificial Anode) Cathodic Protection (SACP): This method uses sacrificial anodes made of a more reactive metal (such as zinc, aluminum, or magnesium) that corrode in place of the protected structure. This process naturally flows because the sacrificial anode is more anodic than the structure.
- Impressed Current Cathodic Protection (ICCP): This method uses an external power source to drive current from an inert anode (such as a titanium mixed-metal oxide or graphite anode) to the metal structure. It is used when galvanic protection is insufficient, such as for large structures.
2. Pourbaix Diagram Explanation
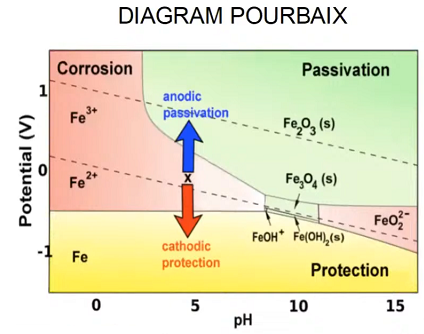
The Pourbaix diagram, also known as a potential-pH diagram, helps understand the electrochemical stability of metals in various environments. It illustrates regions where a metal is stable, corrodes, or passivates based on the pH of the environment and the electrode potential.
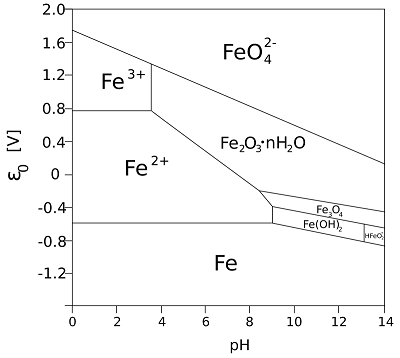
In the context of cathodic protection:
- Corrosion Zone: In this region, the metal oxidizes (loses electrons) and forms soluble ions, leading to material degradation. This zone is where corrosion occurs, and CP is needed.
- Passivation Zone: The metal forms a protective oxide film, which reduces the corrosion rate. CP may not be necessary in this zone, but passivation can be unreliable in fluctuating environmental conditions.
- Immunity Zone: In this zone, the metal is stable, and no corrosion occurs. Cathodic protection aims to shift the operating conditions of the metal to this region by lowering its potential through the application of an external current.
The Pourbaix diagram is particularly useful when designing cathodic protection systems because it helps identify the potential ranges where CP will be most effective for a given material in specific environments.
3. Cathodic Protection Standards
Several international and national standards guide the design, installation, and maintenance of cathodic protection systems. Some key standards include:
- NACE SP0169 (formerly RP0169): “Control of External Corrosion on Underground or Submerged Metallic Piping Systems” – This is one of the most widely used standards for pipeline protection.
- ISO 15589-1: “Petroleum and natural gas industries—Cathodic protection of pipeline systems” – This covers cathodic protection for buried or submerged pipelines.
- ISO 12696: “Cathodic Protection of Steel in Concrete” – Provides guidelines for cathodic protection in reinforced concrete structures.
- BS EN 12954: “Cathodic Protection of Buried or Immersed Metallic Structures” – This is a European standard outlining CP methods for various infrastructure.
- DNVGL-RP-B401: “Cathodic Protection Design” – A recommended practice by DNV GL for the protection of offshore structures, including floating platforms and subsea installations.
These standards ensure consistency in the protection methods and provide guidelines for measurement criteria and maintenance.
4. Applications of Cathodic Protection
Cathodic protection is widely used in various industries:
- Pipelines: CP is extensively used to protect buried and submerged pipelines, especially in oil and gas industries, where the environment is highly corrosive.
- Storage Tanks: Underground storage tanks for fuels and chemicals are prone to corrosion, making CP crucial for long-term integrity.
- Offshore Structures: Platforms, jetties, and ship hulls are constantly exposed to seawater, which is highly corrosive. ICCP systems are usually deployed for these large structures.
- Reinforced Concrete Structures: Steel embedded in concrete can corrode due to the ingress of chlorides or carbonation. CP is applied to prevent structural failure.
- Marine Applications: Boats, docks, and other marine structures use CP to protect against the aggressive marine environment.
5. Measurement Parameters in Cathodic Protection
To ensure cathodic protection is effective, several key parameters need to be monitored and measured:
- Potential (mV): This is the most crucial parameter in CP systems. The structure-to-electrolyte potential is measured using a reference electrode (usually a copper/copper sulfate electrode for soil environments or silver/silver chloride for seawater). The potential should shift towards the negative (cathodic) direction to indicate proper protection.
- Current Density (A/m²): This refers to the amount of current delivered per unit area of the structure. Adequate current density ensures uniform protection across the structure.
- pH and Temperature: Environmental factors like pH and temperature influence the corrosion rate and the performance of the CP system.
- Anode Consumption Rate: In SACP systems, monitoring the consumption rate of sacrificial anodes is essential for planning replacements and ensuring ongoing protection.
- Polarization: The level of polarization indicates how well the metal is being protected. Over-polarization may cause issues like hydrogen embrittlement, especially in high-strength steels.
6. Good vs. Bad Readings during Site Inspection
During site inspections, CP system readings are taken to ensure that the system is functioning correctly. Here’s what constitutes good and bad readings:
- Good Readings:
- Structure-to-Electrolyte Potential: A typical protection potential is between -850 mV to -950 mV for steel pipelines (measured with a copper/copper sulfate electrode in soil environments). This indicates that the structure is sufficiently cathodic and protected from corrosion.
- Uniform Potential Distribution: The readings should be consistent across the structure, indicating even current distribution.
- Low Anode Consumption (SACP): The rate of anode consumption should align with design expectations, ensuring that the sacrificial anodes are not depleted too quickly.
- Stable Readings over Time: CP system potential measurements should remain stable, showing consistent protection over time.
- Bad Readings:
- Structure Potential Too Positive (> -850 mV): This indicates under-protection, and corrosion may still be occurring.
- Excessively Negative Potential (< -1100 mV): This could mean over-protection, which can lead to hydrogen embrittlement, particularly in high-strength steels.
- Significant Potential Fluctuations: Fluctuations may indicate an issue with the CP system or environmental factors affecting the measurements.
- Rapid Anode Depletion (SACP): This could indicate that the sacrificial anodes are undersized or there is an unexpectedly high corrosion rate.
7. Critical Considerations and Best Practices
- Proper Design: Both SACP and ICCP systems require proper design, ensuring that the anode placement, size, and current capacity are appropriate for the structure and environmental conditions.
- Regular Maintenance and Monitoring: CP systems require continuous monitoring to ensure the system operates within specified parameters. Regular potential readings and inspections are critical for detecting issues early.
- Coating and CP Synergy: Combining high-quality coatings with CP systems provides the best results. The coating reduces the current demand, making the CP system more efficient.
- Environmental Considerations: Soil resistivity, water chemistry, and temperature should be thoroughly evaluated, as they significantly influence CP effectiveness.
Conclusion
Cathodic protection is an essential tool for protecting metal structures in aggressive environments from corrosion. A thorough understanding of the principles, supported by standards like NACE SP0169 and ISO 15589-1, ensures that systems are designed and maintained properly. Site inspections using potential measurements are key to verifying protection, and proper maintenance is critical for long-term integrity.
The use of CP systems has been proven to extend the life of pipelines, storage tanks, marine structures, and more, making it an indispensable technology for industries like oil and gas, marine engineering, and infrastructure development.
Recommended Readings
- “Peabody’s Control of Pipeline Corrosion” by A.W. Peabody – A comprehensive guide to corrosion control and cathodic protection.
- “Cathodic Protection of Steel in Concrete and Masonry” by Paul M. Chess – Explores CP applications in reinforced concrete structures.
- “NACE SP0169: Control of External Corrosion on Underground or Submerged Metallic Piping Systems” – A must-read standard for anyone involved in pipeline CP.
These resources will deepen your understanding and provide more insights into the practical aspects of cathodic protection.

Maintenance, projects, and engineering professionals with more than 15 years experience working on power plants, oil and gas drilling, renewable energy, manufacturing, and chemical process plants industries.










